LIANA x Tensor-cell2cell Quickstart (Python)
This tutorial provides an abbreviated version of Tutorials 01-06 in order to give a quick overview of the pipeline to go from a raw counts matrix to downstream cell-cell communication (CCC) analyses. By combining LIANA and Tensor-cell2cell, we get a general framework that can robustly incorporate many existing CCC inference tools, ultimately retriving consensus communication scores for any sample and analyzing all those samples together to identify context-dependent communication programs.
Initial Setups
Enabling GPU use
First, if you are using a NVIDIA GPU with CUDA cores, set use_gpu=True and enable PyTorch with the following code block. Otherwise, set use_gpu=False or skip this part
[1]:
use_gpu = True
if use_gpu:
import tensorly as tl
tl.set_backend('pytorch')
[2]:
import torch
[3]:
torch.cuda.is_available()
[3]:
True
Libraries
Then, import all the packages we will use in this tutorial:
[4]:
import cell2cell as c2c
import liana as li
import decoupler as dc
import scanpy as sc
import plotnine as p9
import seaborn as sns
import matplotlib.pyplot as plt
%matplotlib inline
[5]:
import logging
logging.disable(logging.WARNING) # This is to avoid too many logs because of GSEApy
import warnings
warnings.filterwarnings('ignore')
Directories
Afterwards, specify the data and output directories:
[6]:
output_folder = '../../data/quickstart/outputs_pbmcs/'
c2c.io.directories.create_directory(output_folder)
../../data/quickstart/outputs_pbmcs/ already exists.
Loading Data
We begin by loading the single-cell transcriptomics data. For this tutorial, we will use a dataset of ~25k PBMCs from 8 pooled patient lupus samples, each before and after IFN-beta stimulation (Kang et al., 2018; GSE96583). We use a convenient function to download the data and store it in the AnnData format, on which the scanpy package is built.
[7]:
adata = li.testing.datasets.kang_2018()
Preprocess Expression
Note, we do not include a batch correction step as Tensor-cell2cell can get robust decomposition results without this; however, we have extensive analysis and discuss regarding this topic in Supplementary Tutorial 01
Quality-Control Filtering
The loaded data has already been pre-processed to a degree and comes with cell annotations. Nevertheless, let’s highlight some of the key steps. To ensure that any noisy cells or features are removed, we filter any non-informative cells and genes:
[8]:
# basic filters
sc.pp.filter_cells(adata, min_genes=300)
sc.pp.filter_genes(adata, min_cells=3)
We additionally remove high mitochondrial content:
[9]:
# filter low quality cells with standard QC metrics
adata.var['mt'] = adata.var_names.str.startswith('MT-') # annotate the group of mitochondrial genes as 'mt'
sc.pp.calculate_qc_metrics(adata,
qc_vars=['mt'],
percent_top=None,
log1p=False,
inplace=True)
adata = adata[adata.obs.pct_counts_mt < 15, :]
[10]:
adata
[10]:
View of AnnData object with n_obs × n_vars = 24297 × 15688
obs: 'nCount_RNA', 'nFeature_RNA', 'tsne1', 'tsne2', 'condition', 'cluster', 'cell_type', 'patient', 'nCount_SCT', 'nFeature_SCT', 'integrated_snn_res.0.4', 'seurat_clusters', 'sample', 'cell_abbr', 'n_genes', 'n_genes_by_counts', 'total_counts', 'total_counts_mt', 'pct_counts_mt'
var: 'name', 'n_cells', 'mt', 'n_cells_by_counts', 'mean_counts', 'pct_dropout_by_counts', 'total_counts'
obsm: 'X_pca', 'X_umap'
layers: 'counts'
Normalization
Normalized counts are usually obtained in two essential steps, the first being count depth scaling which ensures that the measured count depths are comparable across cells. This is then usually followed up with log1p transformation, which essentially stabilizes the variance of the counts and enables the use of linear metrics downstream:
[11]:
# save the raw counts to a layer
adata.layers["counts"] = adata.X.copy()
# Normalize the data
sc.pp.normalize_total(adata)
sc.pp.log1p(adata)
Let’s see what our data looks like:
[12]:
adata.obs.head()
[12]:
| nCount_RNA | nFeature_RNA | tsne1 | tsne2 | condition | cluster | cell_type | patient | nCount_SCT | nFeature_SCT | integrated_snn_res.0.4 | seurat_clusters | sample | cell_abbr | n_genes | n_genes_by_counts | total_counts | total_counts_mt | pct_counts_mt | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| index | |||||||||||||||||||
| AAACATACATTTCC-1 | 3017.0 | 877 | -27.640373 | 14.966629 | ctrl | 9 | CD14+ Monocytes | patient_1016 | 1704.0 | 711 | 1 | 1 | ctrl&1016 | CD14 | 877 | 877 | 3017.0 | 0.0 | 0.0 |
| AAACATACCAGAAA-1 | 2481.0 | 713 | -27.493646 | 28.924885 | ctrl | 9 | CD14+ Monocytes | patient_1256 | 1614.0 | 662 | 1 | 1 | ctrl&1256 | CD14 | 713 | 713 | 2481.0 | 0.0 | 0.0 |
| AAACATACCATGCA-1 | 703.0 | 337 | -10.468194 | -5.984389 | ctrl | 3 | CD4 T cells | patient_1488 | 908.0 | 337 | 6 | 6 | ctrl&1488 | CD4T | 337 | 337 | 703.0 | 0.0 | 0.0 |
| AAACATACCTCGCT-1 | 3420.0 | 850 | -24.367997 | 20.429285 | ctrl | 9 | CD14+ Monocytes | patient_1256 | 1738.0 | 653 | 1 | 1 | ctrl&1256 | CD14 | 850 | 850 | 3420.0 | 0.0 | 0.0 |
| AAACATACCTGGTA-1 | 3158.0 | 1111 | 27.952170 | 24.159738 | ctrl | 4 | Dendritic cells | patient_1039 | 1857.0 | 928 | 12 | 12 | ctrl&1039 | DCs | 1111 | 1111 | 3158.0 | 0.0 | 0.0 |
Define columns to use for downstream analysis:
[13]:
sample_key = 'sample'
condition_key = 'condition'
groupby = 'cell_abbr'
Deciphering Cell-Cell Communication
we will use LIANA to infer the ligand-receptor interactions for each sample. LIANA is highly modularized and it natively implements the formulations of a number of methods, including CellPhoneDBv2, Connectome, log2FC, NATMI, SingleCellSignalR, CellChat, a geometric mean, as well as a consensus in the form of a rank aggregate from any combination of methods
[14]:
li.method.show_methods()
[14]:
| Method Name | Magnitude Score | Specificity Score | Reference | |
|---|---|---|---|---|
| 0 | CellPhoneDB | lr_means | cellphone_pvals | Efremova, M., Vento-Tormo, M., Teichmann, S.A.... |
| 0 | Connectome | expr_prod | scaled_weight | Raredon, M.S.B., Yang, J., Garritano, J., Wang... |
| 0 | log2FC | None | lr_logfc | Dimitrov, D., Türei, D., Garrido-Rodriguez, M.... |
| 0 | NATMI | expr_prod | spec_weight | Hou, R., Denisenko, E., Ong, H.T., Ramilowski,... |
| 0 | SingleCellSignalR | lrscore | None | Cabello-Aguilar, S., Alame, M., Kon-Sun-Tack, ... |
| 0 | CellChat | lr_probs | cellchat_pvals | Jin, S., Guerrero-Juarez, C.F., Zhang, L., Cha... |
| 0 | Rank_Aggregate | magnitude_rank | specificity_rank | Dimitrov, D., Türei, D., Garrido-Rodriguez, M.... |
| 0 | Geometric Mean | lr_gmeans | gmean_pvals | CellPhoneDBv2's permutation approach applied t... |
LIANA classifies the scoring functions from the different methods into two categories: those that infer the “Magnitude” and “Specificity” of interactions. We define the “Magnitude” of an interaction as a measure of the strength of the interaction’s expression, and the “Specificity” of an interaction is a measure of how specific an interaction is to a given pair of clusters. Generally, these categories are complementary, and the magnitude of the interaction is often a proxy of the specificity of the interaction. For example, a ligand-receptor interaction with a high magnitude score in a given pair of cell types is likely to also be specific, and vice versa.
When considering ligand-receptor prior knowledge resources, a common theme is the trade-off between coverage and quality, and similarly each resource comes with its own biases. In this regard, LIANA builds on OmniPath29 as any of the resources in LIANA are obtained via OmniPath. These include the expert-curated resources of CellPhoneDBv223, CellChat27, ICELLNET30, connectomeDB202025, CellTalkDB31, as well as 10 others. LIANA further provides a consensus expert-curated resource from the aforementioned five resources, along with some curated interactions from SignaLink. In this protocol, we will use the consensus resource from liana, though any of the other resources are available via LIANA, and one can also use liana with their own custom resource.
[15]:
liana_resources = li.resource.show_resources()
print(*liana_resources, sep = ', ')
baccin2019, cellcall, cellchatdb, cellinker, cellphonedb, celltalkdb, connectomedb2020, consensus, embrace, guide2pharma, hpmr, icellnet, italk, kirouac2010, lrdb, mouseconsensus, ramilowski2015
Selecting any of the lists of ligand-receptor pairs in LIANA can be done through the following command (here we select the aforementioned “consensus” resource:
[16]:
lr_pairs = li.resource.select_resource('consensus')
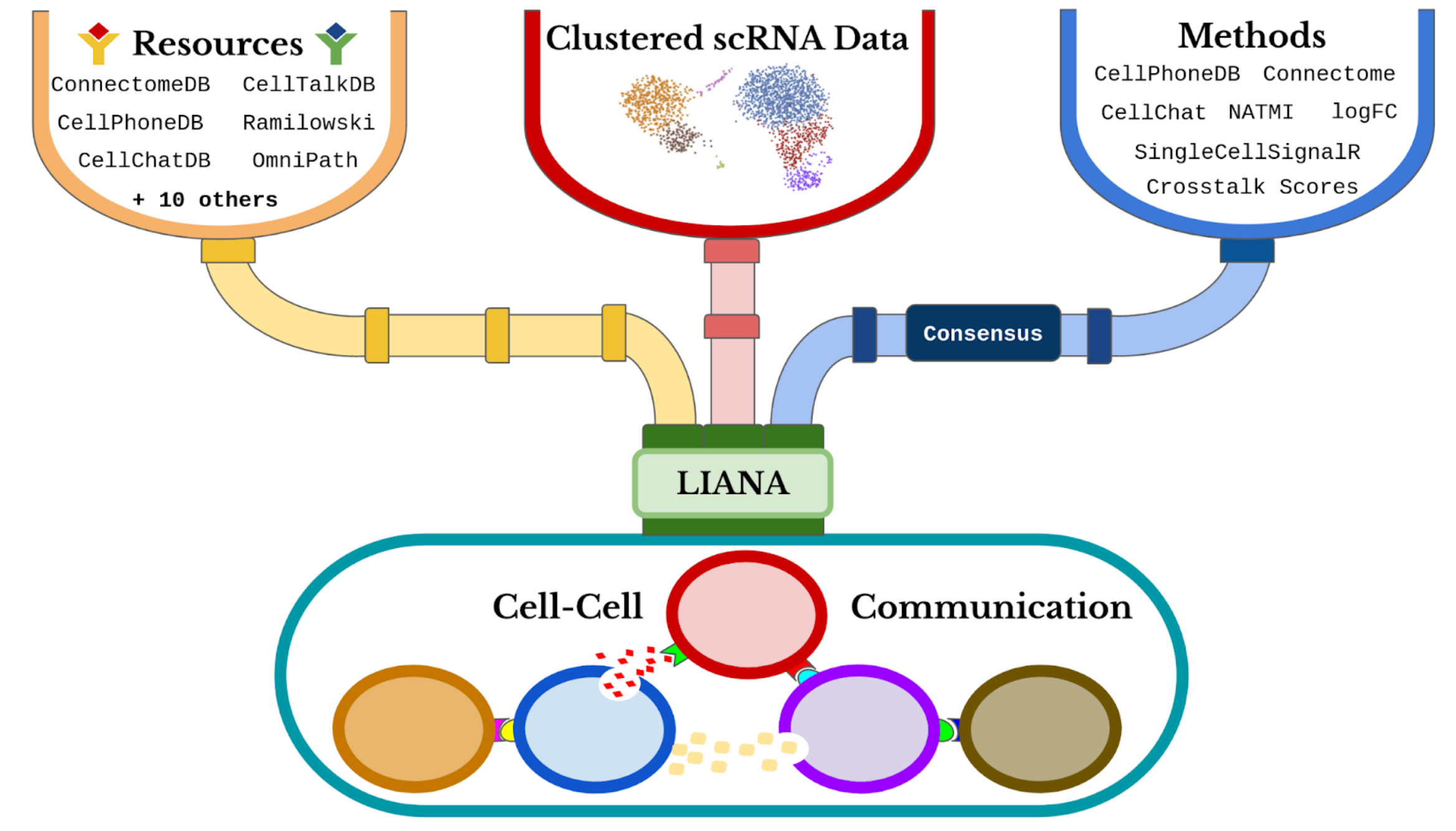
Next, we can run LIANA on each sample across the available methods. By default, LIANA calculates an aggregate rank across these mtehods using a re-implementation of the RobustRankAggregate method, and generates a probability distribution for ligand-receptors that are ranked consistently better than expected under a null hypothesis (See Appendix 2). The consensus of ligand-receptor interactions across methods can therefore be treated as a p-value.
[17]:
li.mt.rank_aggregate.by_sample(adata,
sample_key=sample_key,
groupby=groupby,
resource_name = 'consensus',
expr_prop=0.1, # must be expressed in expr_prop fraction of cells
min_cells = 5,
n_perms = 100,
use_raw = False, # run on log- and library-normalized counts
verbose = True,
inplace = True,
return_all_lrs=True, # return all LR values
)
Now running: stim&1488: 100%|███████████████████████████████████████| 16/16 [01:20<00:00, 5.01s/it]
The parameterrs used here are as follows:
adatastands for Anndata, and we pass here with an object with a single sample/context.resource_nameis the name of any of the resources available in lianagroupbycorresponds to the cell group label stored inadata.obs.use_rawis boolean that indicates whether to use theadata.rawslot, here the log-normalized counts are assigned toadata.X, other options include passing the name of a layer via thelayerparameter or using the counts stored inadata.raw.expr_propis the expression proportion threshold (in terms of cells per cell type expressing the protein)threshold of expression for any protein subunit involved in the interaction, according to which we keep or discard the interactions.min_cellsis the minimum number of cells per cell type required for a cell type to be considered in the analysisverboseis a boolean that indicates whether to print the progress of the functioninplaceis a boolean that indicates whether storing the results in place, i.e. toadata.uns[“liana_res”].
Let’s see what the results look like:
[18]:
adata.uns['liana_res'].head()
[18]:
| sample | source | target | ligand_complex | receptor_complex | lr_means | cellphone_pvals | expr_prod | scaled_weight | lr_logfc | spec_weight | lrscore | lr_probs | cellchat_pvals | specificity_rank | magnitude_rank | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | ctrl&101 | FGR3 | CD14 | TIMP1 | CD63 | 2.634805 | 0.0 | 6.247571 | 1.544213 | 2.285625 | 0.132291 | 0.984058 | 0.268787 | 0.0 | 6.401877e-09 | 1.325768e-12 |
| 1 | ctrl&101 | FGR3 | DCs | TIMP1 | CD63 | 2.321779 | 0.0 | 4.076271 | 1.154960 | 1.413446 | 0.086314 | 0.980338 | 0.199095 | 0.0 | 8.764034e-09 | 8.484083e-11 |
| 2 | ctrl&101 | CD14 | CD14 | TIMP1 | CD63 | 2.261914 | 0.0 | 4.904150 | 1.277493 | 1.908720 | 0.103844 | 0.982043 | 0.225244 | 0.0 | 6.401877e-09 | 1.548535e-10 |
| 3 | ctrl&101 | NK | CD8T | B2M | CD3D | 2.319840 | 0.0 | 2.631051 | 1.018839 | 0.661349 | 0.079802 | 0.975643 | 0.143878 | 0.0 | 1.773842e-08 | 9.029901e-10 |
| 4 | ctrl&101 | NK | NK | B2M | KLRD1 | 2.337043 | 0.0 | 2.767930 | 1.685281 | 0.911614 | 0.096133 | 0.976239 | 0.136489 | 0.0 | 6.401877e-09 | 9.662321e-10 |
This dataframe provides the results from running each method, as well as the consensus scores across the methods. In our case, we are interested in the magnitude consensus scored denoted by the 'magnitude_rank' column.
We can visualize the output as a dotplot across multiple samples:
[49]:
plot = li.pl.dotplot_by_sample(adata=adata,
colour='magnitude_rank',
size='specificity_rank',
source_labels=["CD4T", "B", "FGR3"],
target_labels=["CD8T", 'DCs', 'CD14'],
ligand_complex = 'B2M',
receptor_complex=["KLRD1", "LILRB2", "CD3D"],
sample_key=sample_key,
inverse_colour=True,
inverse_size=True,
figure_size=(12, 9),
size_range=(1, 6),
)
plot.save(output_folder + '/Dotplot-by-sample.pdf', height=9, width=12)
plot
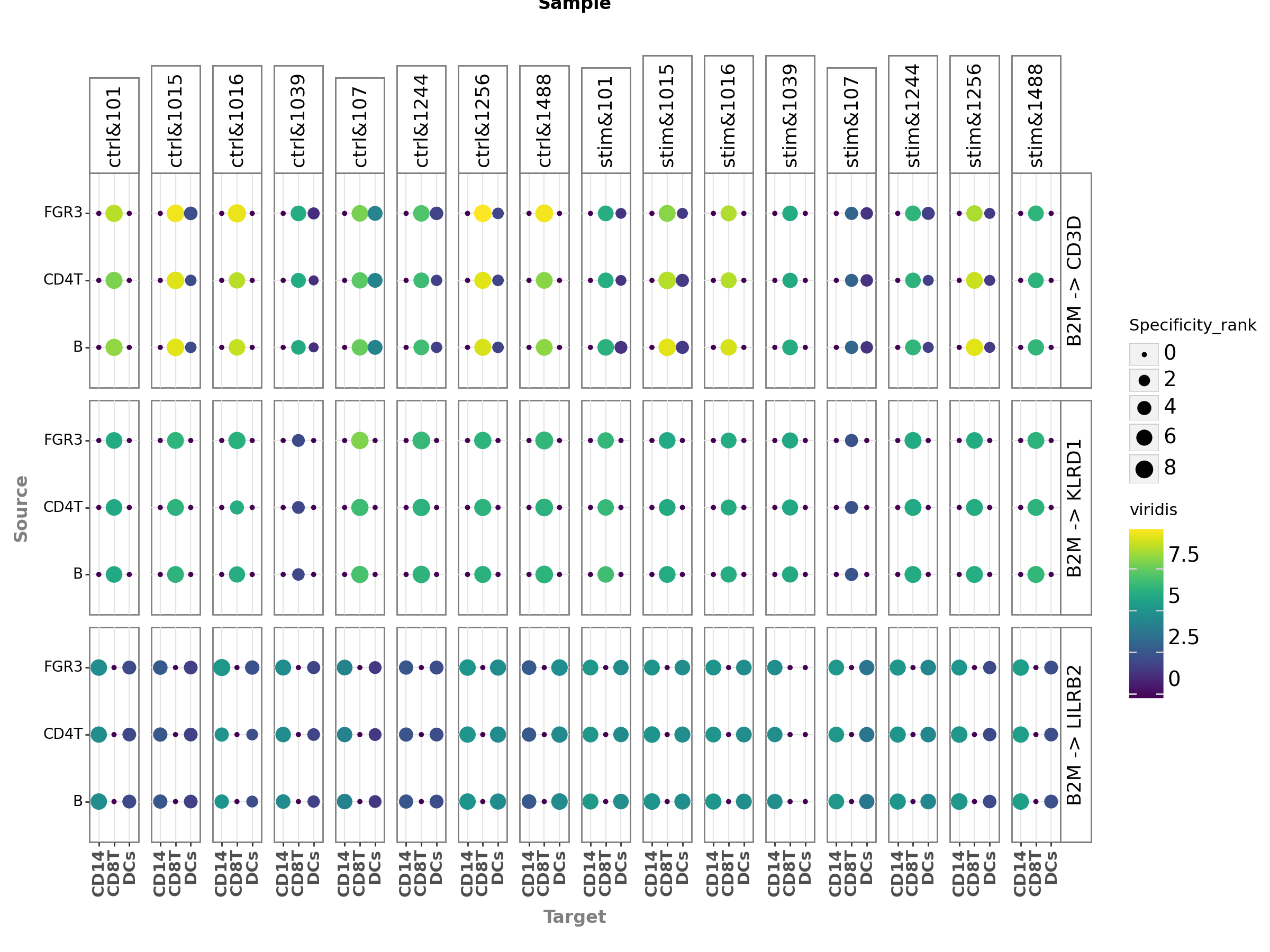
[49]:
<Figure Size: (1200 x 900)>
The key parameters used here are:
source_labelsis a list containing the names of the sender cells of interest.target_labelsis a list containing the names of the receiver cells of interest.ligand_complexis a list containing the names of the ligands of interest.receptor_complexis a list containing the names of the receptors of interest.sample_keyis a string containing the column name where samples are specified.
Let’s export it in case we want to access it later:
[19]:
adata.uns['liana_res'].to_csv(output_folder + '/LIANA_by_sample.csv', index=False)
Alternatively, one could just export the whole AnnData object, together with the ligand-receptor results stored:
[20]:
adata.write_h5ad(output_folder + '/adata_processed.h5ad', compression='gzip')
Comparing cell-cell communication across multiple samples
We can use Tensor-cell2cell to infer context-dependent CCC patterns from multiple samples simultaneously. To do so, we must first restructure the communication scores (LIANA’s output) into a 4D-Communication Tensor.
The tensor is built as follows: we create matrices with the communication scores for each of the ligand-receptor pairs within the same sample, then generate a 3D tensor for each sample, and finally concatenate them to form the 4D tensor:
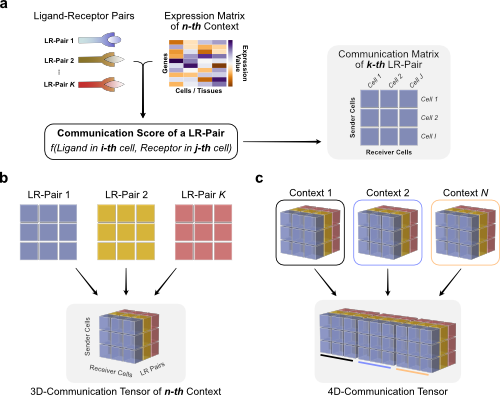
First, we generate a list containing all samples from our AnnData object. Here, we can find the names of each of the samples in the sample column of the adata.obs information:
[21]:
sorted_samples = sorted(adata.obs[sample_key].unique())
Then we can directly pass the communication scores from LIANA to build the 3D tensors for each sample (panel c in last figure), and concatenate them, with the following function:
[22]:
adata.uns['liana_res'].head()
[22]:
| sample | source | target | ligand_complex | receptor_complex | lr_means | cellphone_pvals | expr_prod | scaled_weight | lr_logfc | spec_weight | lrscore | lr_probs | cellchat_pvals | specificity_rank | magnitude_rank | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | ctrl&101 | FGR3 | CD14 | TIMP1 | CD63 | 2.634805 | 0.0 | 6.247571 | 1.544213 | 2.285625 | 0.132291 | 0.984058 | 0.268787 | 0.0 | 6.401877e-09 | 1.325768e-12 |
| 1 | ctrl&101 | FGR3 | DCs | TIMP1 | CD63 | 2.321779 | 0.0 | 4.076271 | 1.154960 | 1.413446 | 0.086314 | 0.980338 | 0.199095 | 0.0 | 8.764034e-09 | 8.484083e-11 |
| 2 | ctrl&101 | CD14 | CD14 | TIMP1 | CD63 | 2.261914 | 0.0 | 4.904150 | 1.277493 | 1.908720 | 0.103844 | 0.982043 | 0.225244 | 0.0 | 6.401877e-09 | 1.548535e-10 |
| 3 | ctrl&101 | NK | CD8T | B2M | CD3D | 2.319840 | 0.0 | 2.631051 | 1.018839 | 0.661349 | 0.079802 | 0.975643 | 0.143878 | 0.0 | 1.773842e-08 | 9.029901e-10 |
| 4 | ctrl&101 | NK | NK | B2M | KLRD1 | 2.337043 | 0.0 | 2.767930 | 1.685281 | 0.911614 | 0.096133 | 0.976239 | 0.136489 | 0.0 | 6.401877e-09 | 9.662321e-10 |
[23]:
# build the tensor
tensor = li.multi.to_tensor_c2c(liana_res=adata.uns['liana_res'], # LIANA's dataframe containing results
sample_key=sample_key, # Column name of the samples
source_key='source', # Column name of the sender cells
target_key='target', # Column name of the receiver cells
ligand_key='ligand_complex', # Column name of the ligands
receptor_key='receptor_complex', # Column name of the receptors
score_key='magnitude_rank', # Column name of the communication scores to use
inverse_fun=lambda x: 1 - x, # Transformation function
how='outer_cells', # What to include across all samples
outer_fraction=1/3, # Fraction of samples as threshold to include cells and LR pairs.
context_order=sorted_samples, # Order to store the contexts in the tensor
)
100%|███████████████████████████████████████████████████████████████| 16/16 [00:21<00:00, 1.36s/it]
The key parameters when building a tensor are:
liana_resis the dataframe containing the results from LIANA, usually located in `adata.uns[‘liana_res’]. We can pass directly the AnnData object to the parameter adata in this function. If the AnnData object is passed, we do not need to specify the liana_res parameter.sample_key,source_key,target_key,ligand_key,receptor_key, andscore_keyare the column names in the dataframe containing the samples, sender cells, receiver cells, ligands, receptors, and communication scores, respectively. Each row of the dataframe contains a unique combination of these elements.inverse_funis the function we use to convert the communication score before using it to build the tensor. In this case, the ‘magnitude_rank’ score generated by LIANA considers low values as the most important ones, ranging from 0 to 1. In contrast, Tensor-cell2cell requires higher values to be the most important scores, so here we pass a function (lambda x: 1 - x) to adapt LIANA’s magnitude-rank scores (subtracts the LIANA’s score from 1). IfNoneis passed instead, no transformation will be performed on the communication score. If using other scores coming from one of the methods implemented in LIANA, a similar transformation can be done depending on the parameters and assumptions of the scoring method.howcontrols which ligand-receptor pairs and cell types to include when building the tensor. This decision depends on whether the missing values across a number of samples for both ligand-receptor interactions and sender-receiver cell pairs are considered to be biologically-relevant. Options are:'inner'is the more strict option since it only considers only cell types and LR pairs that are present in all contexts (intersection).'outer'considers all cell types and LR pairs that are present across contexts (union).'outer_lrs'considers only cell types that are present in all contexts (intersection), while all LR pairs that are present across contexts (union).'outer_cells'considers only LR pairs that are present in all contexts (intersection), while all cell types that are present across contexts (union).
outer_fractioncontrols the elements to include in the union scenario of thehowoptions. Only elements that are present at least in this fraction of samples/contexts will be included. When this value is 0, the tensor includes all elements across the samples. When this value is 1, it acts as usinghow='inner'.context_orderis a list specifying the order of the samples. The order of samples does not affect the results, but it is useful for posterior visualizations.
We can check the shape of this tensor to verify the number of samples, LR pairs, sender cells, adn receiver cells, respectively:
[24]:
tensor.shape
[24]:
torch.Size([16, 435, 7, 7])
In addition, optionally we can generate the metadata for coloring the elements in each of the tensor dimensions (i.e., for each of the contexts/samples, ligand-receptor pairs, sender cells, and receiver cells). For this, we have to pass dictionaries for each dimension containing the elements and their respective major groups. In cases where we do not account with such information, we do not need to generate such dictionaries. For the contexts/samples dictionary, we can use the metadata in the AnnData object. This information will be used later for visualization of the results.
In this example dataset, we can find samples in the column 'sample', while the condition groups are found in the column 'condition':
[25]:
context_dict = adata.obs.sort_values(by=sample_key) \
.set_index(sample_key)[condition_key] \
.to_dict()
Then, the metadata can be generated with:
[26]:
dimensions_dict = [context_dict, None, None, None]
meta_tensor = c2c.tensor.generate_tensor_metadata(interaction_tensor=tensor,
metadata_dicts=dimensions_dict,
fill_with_order_elements=True
)
We can export our tensor and its metadata:
[27]:
c2c.io.export_variable_with_pickle(variable=tensor,
filename=output_folder + '/Tensor.pkl')
c2c.io.export_variable_with_pickle(variable=meta_tensor,
filename=output_folder + '/Tensor-Metadata.pkl')
../../data/quickstart/outputs_pbmcs//Tensor.pkl was correctly saved.
../../data/quickstart/outputs_pbmcs//Tensor-Metadata.pkl was correctly saved.
Then, we can load them with:
[28]:
tensor = c2c.io.read_data.load_tensor(output_folder + '/Tensor.pkl')
meta_tensor = c2c.io.load_variable_with_pickle(output_folder + '/Tensor-Metadata.pkl')
Perform Tensor Factorization
Now that we have built the tensor and its metadata, we can run Tensor Component Analysis via Tensor-cell2cell with one simple command that we implemented for our unified framework:
[29]:
c2c.analysis.run_tensor_cell2cell_pipeline(tensor,
meta_tensor,
rank=None, # Number of factors to perform the factorization. If None, it is automatically determined by an elbow analysis
tf_optimization='regular', # To define how robust we want the analysis to be.
random_state=0, # Random seed for reproducibility
device='cuda', # Device to use. If using GPU and PyTorch, use 'cuda'. For CPU use 'cpu'
output_folder=output_folder, # Whether to save the figures in files. If so, a folder pathname must be passed
)
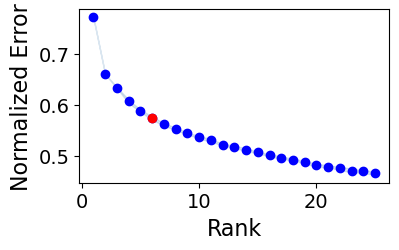
Running Elbow Analysis
100%|███████████████████████████████████████████████████████████████| 25/25 [03:40<00:00, 8.83s/it]
The rank at the elbow is: 6
Running Tensor Factorization
Generating Outputs
Loadings of the tensor factorization were successfully saved into ../../data/quickstart/outputs_pbmcs//Loadings.xlsx
[29]:
<cell2cell.tensor.tensor.PreBuiltTensor at 0x7ffa482c84d0>
Key parameters are:
rankis the number of factors or latent patterns we want to obtain from the analysis. You can either indicate a specific number or leave it asNoneto perform the decomposition with a suggested number from an elbow analysis.tf_optimizationindicates whether running the analysis in the'regular'or the'robust'way. The'regular'way runs the tensor decomposition less number of times than the robust way to select an optimal result. Additionally, the former employs less strict convergence parameters to obtain optimal results than the latter, which is also translated into a faster generation of results. Important: When usingtf_optimization='robust'the analysis takes much longer to run than usingtf_optimization='regular'. However, the latter may generate less robust results.random_stateis the seed for randomization. It controls the randomization used when initializing the optimization algorithm that performs the tensor decomposition. It is useful for reproducing the same result every time that the analysis is run. IfNone, a different randomization will be used each time.deviceindicates whether we are using the'cpu'or a GPU with'cuda'cores.output_folderis the full path to the folder where the results will be saved. Make sure that this folder exists before passing it here.
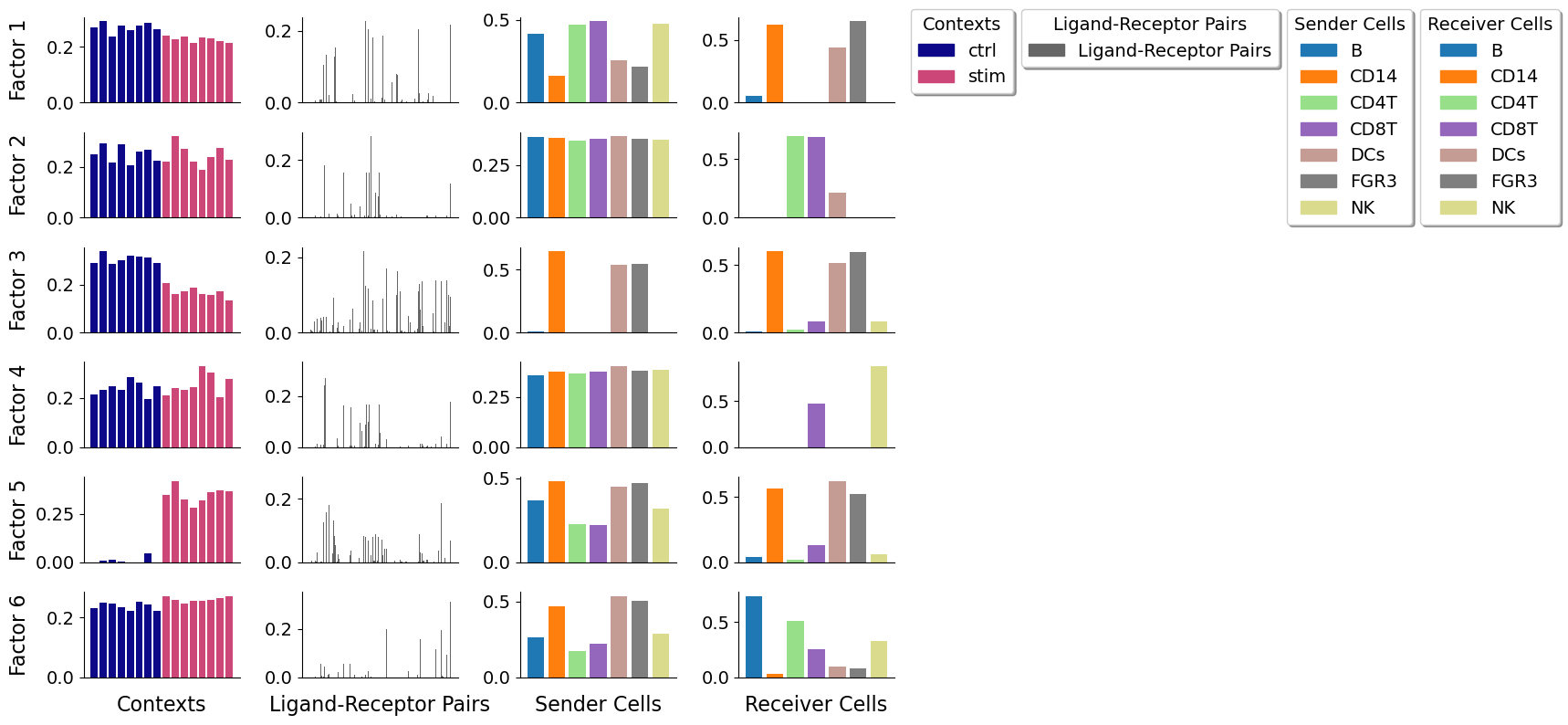
The figure representing the loadings in each factor generated here can be interpreted by interconnecting all dimensions within a single factor. For example, if we take one of these factors, the cell-cell communication program occurs in each sample proportionally to their loadings. Then, this signature can be interpreted with the loadings of the ligand-receptor pairs, sender cells, and receiver cells. Ligands in high-loading ligand-receptor pairs are sent predominantly by high-loading sender cells, and interact with the cognate receptors on the high-loadings receiver cells.
Downstream Visualizations: Making sense of the factors
After running the decomposition, the results are stored in the factors attribute of the tensor object. This attribute is a dictionary containing the loadings for each of the elements in every tensor dimension. Loadings delineate the importance of that particular element to that factor. Keys are the names of the different dimension.
[30]:
tensor.factors.keys()
[30]:
odict_keys(['Contexts', 'Ligand-Receptor Pairs', 'Sender Cells', 'Receiver Cells'])
We can inspect the loadings of the samples, for example, located under the key 'Contexts':
[31]:
tensor.factors['Contexts']
[31]:
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | |
|---|---|---|---|---|---|---|
| ctrl&101 | 0.270332 | 0.247286 | 0.292592 | 0.215103 | 4.010570e-10 | 0.231475 |
| ctrl&1015 | 0.291630 | 0.291086 | 0.341411 | 0.233093 | 1.044283e-02 | 0.250662 |
| ctrl&1016 | 0.238585 | 0.217481 | 0.288124 | 0.247691 | 1.342540e-02 | 0.246392 |
| ctrl&1039 | 0.275843 | 0.289148 | 0.302774 | 0.231855 | 4.795332e-03 | 0.233603 |
| ctrl&107 | 0.259571 | 0.206496 | 0.321550 | 0.283955 | 1.288960e-06 | 0.221600 |
| ctrl&1244 | 0.277072 | 0.260974 | 0.317252 | 0.261879 | 9.721018e-05 | 0.254720 |
| ctrl&1256 | 0.286455 | 0.266752 | 0.315117 | 0.195056 | 4.492147e-02 | 0.244827 |
| ctrl&1488 | 0.265057 | 0.222111 | 0.292968 | 0.249131 | 5.467816e-06 | 0.222546 |
| stim&101 | 0.239308 | 0.220685 | 0.207800 | 0.210447 | 3.497421e-01 | 0.270802 |
| stim&1015 | 0.226724 | 0.319908 | 0.161027 | 0.238773 | 4.213534e-01 | 0.260401 |
| stim&1016 | 0.235833 | 0.270952 | 0.173388 | 0.231356 | 3.268313e-01 | 0.248756 |
| stim&1039 | 0.214386 | 0.219641 | 0.189005 | 0.243921 | 2.827033e-01 | 0.255100 |
| stim&107 | 0.234389 | 0.188312 | 0.160595 | 0.329494 | 3.207296e-01 | 0.255509 |
| stim&1244 | 0.229499 | 0.237026 | 0.155739 | 0.305001 | 3.654820e-01 | 0.258046 |
| stim&1256 | 0.220098 | 0.275639 | 0.174542 | 0.204366 | 3.748907e-01 | 0.265136 |
| stim&1488 | 0.214711 | 0.227280 | 0.136243 | 0.278003 | 3.661750e-01 | 0.273102 |
[32]:
tensor.factors['Sender Cells']
[32]:
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | |
|---|---|---|---|---|---|---|
| B | 0.416015 | 0.385384 | 0.010189 | 0.358390 | 0.367898 | 0.263979 |
| CD14 | 0.164361 | 0.382299 | 0.645374 | 0.376272 | 0.484843 | 0.467544 |
| CD4T | 0.469885 | 0.365400 | 0.000145 | 0.367074 | 0.229020 | 0.175351 |
| CD8T | 0.490371 | 0.374164 | 0.002215 | 0.373320 | 0.220655 | 0.221917 |
| DCs | 0.254085 | 0.388060 | 0.534771 | 0.402502 | 0.452011 | 0.538169 |
| FGR3 | 0.218537 | 0.376688 | 0.545346 | 0.381490 | 0.470661 | 0.507878 |
| NK | 0.475756 | 0.373264 | 0.001327 | 0.385126 | 0.320317 | 0.290097 |
Downstream Visualizations
We can use these loadings to compare pairs of sample major groups with boxplots and statistical tests:
[33]:
fig_filename = output_folder + '/PBMCs-Boxplots.pdf'
_ = c2c.plotting.context_boxplot(context_loadings=tensor.factors['Contexts'],
metadict=context_dict,
nrows=3,
figsize=(16, 12),
statistical_test='t-test_ind',
pval_correction='fdr_bh',
cmap='plasma',
verbose=False,
filename=fig_filename
)
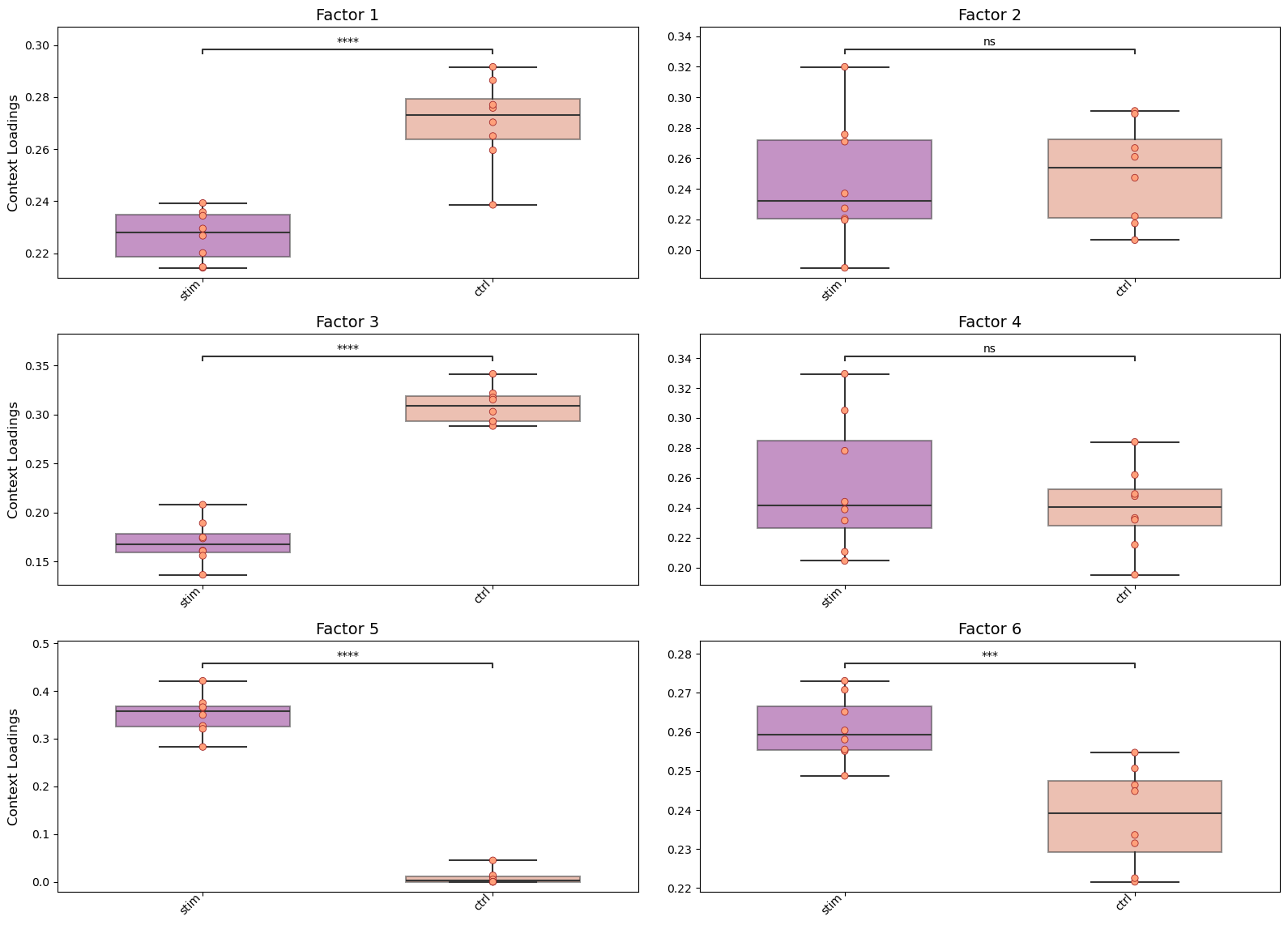
Using the loadings for any dimension, we can also generate heatmaps for the elements with loadings above a certain threshold. Additionally, we can cluster these elements by the similarity of their loadings across all factors:
[34]:
fig_filename = output_folder + '/Clustermap-LRs.pdf'
_ = c2c.plotting.loading_clustermap(loadings=tensor.factors['Ligand-Receptor Pairs'],
loading_threshold=0.1,
use_zscore=False,
figsize=(28, 8),
filename=fig_filename,
row_cluster=False,
tick_fontsize=16,
dendrogram_ratio=0.15,
)
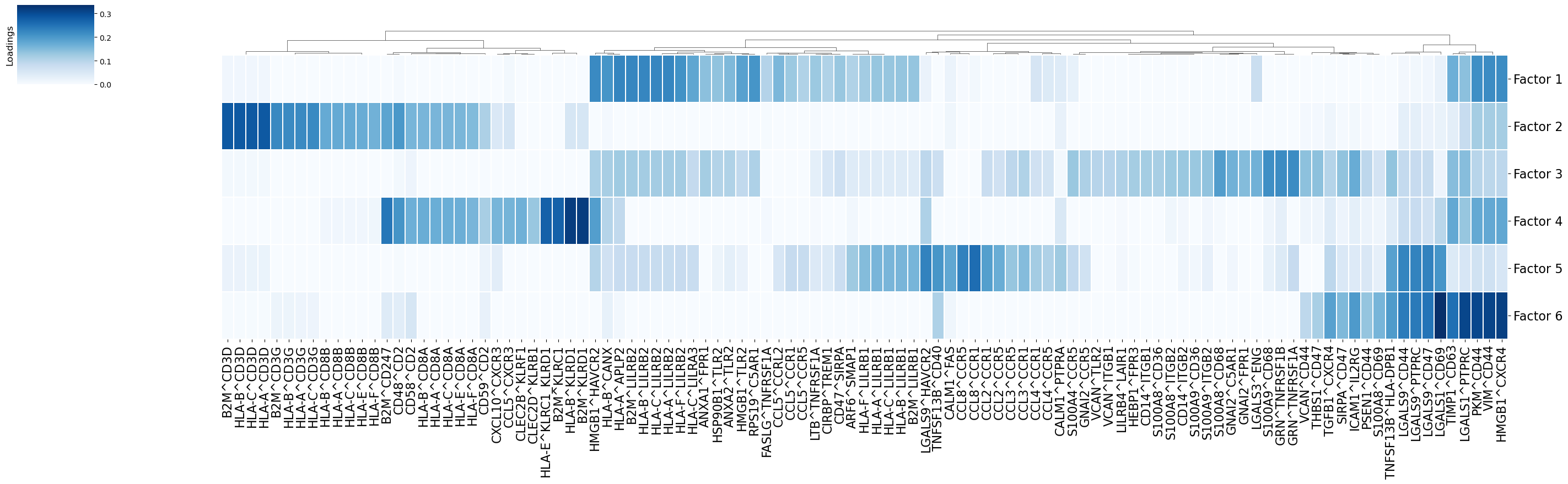
Overall CCI potential: Heatmap and network visualizations of sender-receiver cell pairs
In addition, we can also evaluate the overall interactions between sender-receiver cell pairs that are determinant for a given factor or program. We can do it through a heatmap where the X-axis represent the receiver cells and the Y-axis shows the receiver cells. Here, the potential of interaction is calculated as the outer product between the loadings for the sender and receiver cells dimensions of a particular factor. To illustrate this, we chose Factor 3, but this can be repeated for every factor obtained in the decomposition.
[35]:
selected_factor = 'Factor 3'
[36]:
loading_product = c2c.analysis.tensor_downstream.get_joint_loadings(tensor.factors,
dim1='Sender Cells',
dim2='Receiver Cells',
factor=selected_factor,
)
[37]:
lprod_cm = c2c.plotting.loading_clustermap(loading_product.T, # Remove .T to transpose the axes
use_zscore=False, # Whether standardizing the loadings across factors
figsize=(8, 8),
filename=output_folder + '/Clustermap-CC-Pairs.pdf',
cbar_label='Loading Product',
)
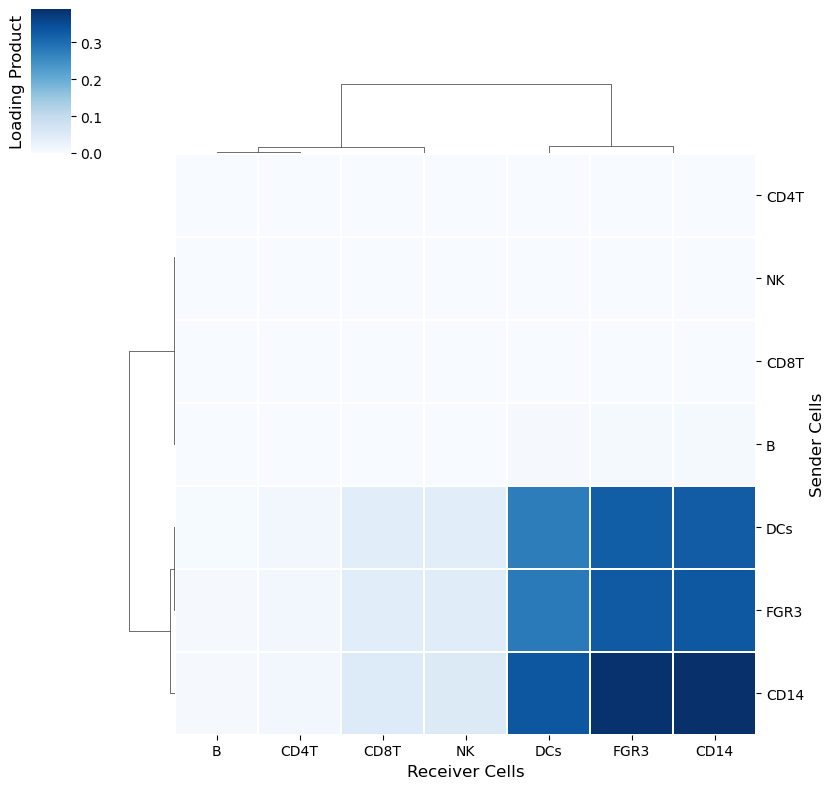
Similarly, an interaction network can be created for each factor by using the loading product between sender and receiver cells. First we need to choose a threshold to indicate what pair of cells are interacting. This can be done as shown in the extended tutorial.
[38]:
threshold = 0.075
Then, we can plot all networks we are interested in:
[39]:
c2c.plotting.ccc_networks_plot(tensor.factors,
included_factors=['Factor 3', 'Factor 4', 'Factor 5'],
ccc_threshold=threshold, # Only important communication
nrows=1,
panel_size=(12,12), # This changes the size of each figure panel.
node_label_size=30,
filename=output_folder + '/Factor-Networks.pdf',
)
[39]:
(<Figure size 3600x1200 with 3 Axes>,
array([<Axes: title={'center': 'Factor 3'}>,
<Axes: title={'center': 'Factor 4'}>,
<Axes: title={'center': 'Factor 5'}>], dtype=object))
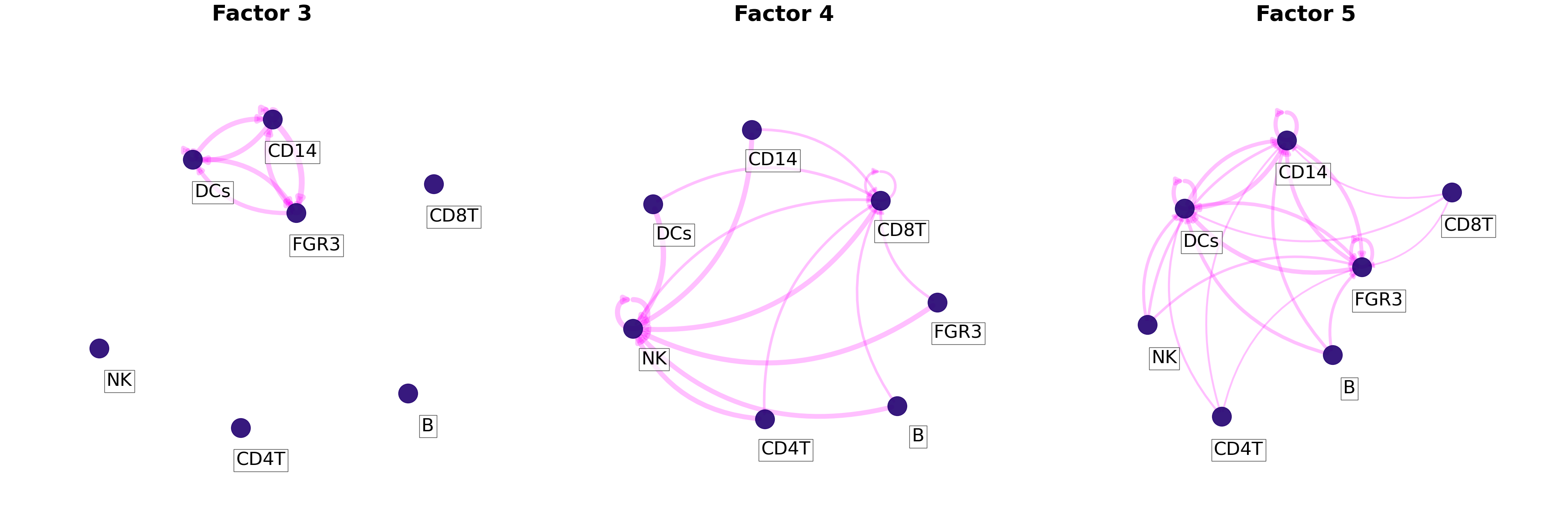
If included_factors=None, all factors will be plotted.
Pathway Enrichment Analysis: Interpreting the context-driven communication
Classical Pathway Enrichment
As the number of inferred interactions increases, the interpretation of the inferred cell-cell communication networks becomes more challenging. To this end, we can perform pathway enrichment analysis to identify the general biological processes that are enriched in the inferred interactions. Here, we will perform classical gene set enrichment analysis with Reactome gene sets.
For the pathway enrichment analysis with GSEA, we use ligand-receptor pairs instead of individual genes. Reactome was initially designed to work with sets of genes, so first we need to generate ligand-receptor sets for each of it’s pathways:
[40]:
lr_loadings = tensor.factors['Ligand-Receptor Pairs']
# Generate list with ligand-receptors pairs in DB
lr_list = ['^'.join(row) for idx, row in lr_pairs.iterrows()]
# Specify the organism and pathway database to use for building the LR set
organism = "human"
pathwaydb = "Reactome"
# Generate ligand-receptor gene sets
lr_set = c2c.external.generate_lr_geneset(lr_list,
complex_sep='_',
lr_sep='^',
organism=organism,
pathwaydb=pathwaydb,
readable_name=True,
output_folder=output_folder
)
Next, we can perform enrichment analysis on each factor using the loadings of the ligand-receptor pairs to obtain the normalized-enrichment scores (NES) and corresponding P-values from GSEA:
[41]:
pvals, scores, gsea_df = c2c.external.run_gsea(loadings=lr_loadings,
lr_set=lr_set,
output_folder=output_folder,
weight=1,
min_size=10,
permutations=999,
processes=6,
random_state=6,
significance_threshold=0.05,
)
../../data/quickstart/outputs_pbmcs/ already exists.
100%|█████████████████████████████████████████████████████████████████| 6/6 [00:01<00:00, 3.52it/s]
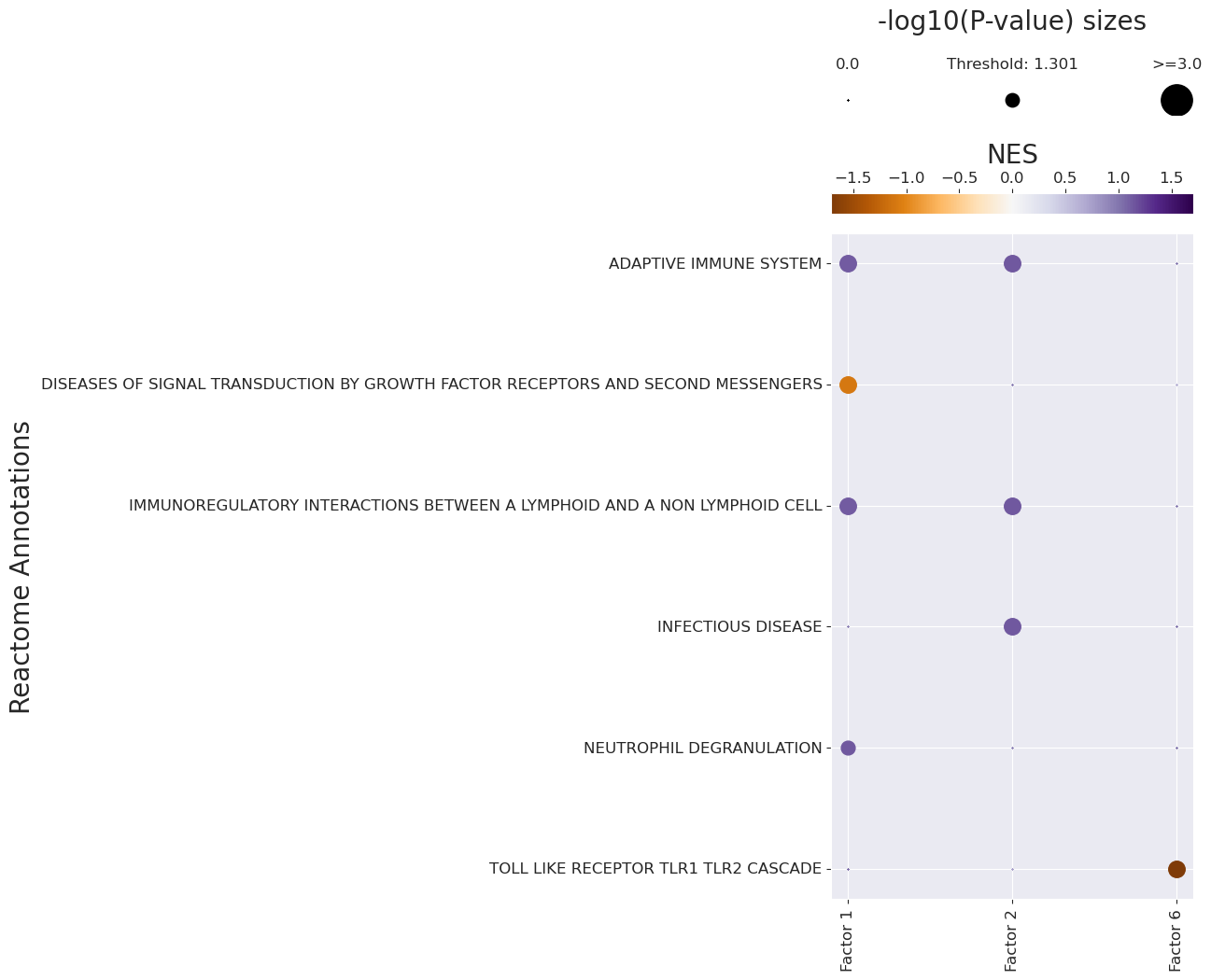
The enriched pathways for each factor are:
[42]:
gsea_df.loc[(gsea_df['Adj. P-value'] <= 0.05) & (gsea_df['NES'] > 0.)]
[42]:
| Factor | Term | NES | P-value | Adj. P-value | |
|---|---|---|---|---|---|
| 0 | Factor 1 | NEUTROPHIL DEGRANULATION | 1.143737 | 0.002002 | 0.046547 |
| 1 | Factor 1 | ADAPTIVE IMMUNE SYSTEM | 1.121092 | 0.001000 | 0.026571 |
| 2 | Factor 1 | IMMUNOREGULATORY INTERACTIONS BETWEEN A LYMPHO... | 1.120429 | 0.001000 | 0.026571 |
| 31 | Factor 2 | INFECTIOUS DISEASE | 1.139834 | 0.001000 | 0.026571 |
| 32 | Factor 2 | IMMUNOREGULATORY INTERACTIONS BETWEEN A LYMPHO... | 1.139245 | 0.001000 | 0.026571 |
| 33 | Factor 2 | ADAPTIVE IMMUNE SYSTEM | 1.135042 | 0.001000 | 0.026571 |
The depleted pathways are:
[43]:
gsea_df.loc[(gsea_df['Adj. P-value'] <= 0.05) & (gsea_df['NES'] < 0.)]
[43]:
| Factor | Term | NES | P-value | Adj. P-value | |
|---|---|---|---|---|---|
| 4 | Factor 1 | DISEASES OF SIGNAL TRANSDUCTION BY GROWTH FACT... | -1.097015 | 0.001 | 0.026571 |
| 155 | Factor 6 | TOLL LIKE RECEPTOR TLR1 TLR2 CASCADE | -1.705882 | 0.001 | 0.026571 |
Finally, we can visualize the enrichment results using a dotplot:
[44]:
pathway_label = '{} Annotations'.format(pathwaydb)
fig_filename = output_folder + '/GSEA-Dotplot.pdf'
with sns.axes_style("darkgrid"):
dotplot = c2c.plotting.pval_plot.generate_dot_plot(pval_df=pvals,
score_df=scores,
significance=0.05,
xlabel='',
ylabel='{} Annotations'.format(pathwaydb),
cbar_title='NES',
cmap='PuOr',
figsize=(5, 12),
label_size=20,
title_size=20,
tick_size=12,
filename=fig_filename
)
Footprint Enrichment
Footprint enrichment analysis build upon classic geneset enrichment analysis, as instead of considering the genes involved in a biological activity, they consider the genes affected by the activity, or in other words the genes that change downstream of said activity (Dugourd and Saez-Rodriguez, 2019).
In this case, we will use the PROGENy pathway resource to perform footprint enrichment analysis. PROGENy was built in a data-driven manner using perturbation and cancer lineage data (Schubert et al, 2019), as a consequence it also assigns different importances or weights to each gene in its pathway genesets. To this end, we need an enrichment method that can take weights into account, and here we will use multi-variate linear models
from the decoupler-py package to perform this analysis (Badia-i-Mompel et al., 2022).
Let’s load the PROGENy genesets and then convert them to sets of weighted ligand-receptor pairs:
[45]:
# We first load the PROGENy gene sets
net = dc.get_progeny(organism='human', top=5000)
# Then convert them to sets with weighed ligand-receptor pairs
lr_progeny = li.rs.generate_lr_geneset(lr_pairs, net, lr_sep="^")
Next, we can run the footprint enrichment analysis:
[46]:
estimate, pvals = dc.run_mlm(lr_loadings.transpose(),
lr_progeny,
source="source",
target="interaction",
use_raw=False)
Finally, we can visualize the results:
[47]:
fig_filename = output_folder + '/PROGENy.pdf'
_ = sns.clustermap(estimate, xticklabels=estimate.columns, cmap='coolwarm', z_score=4)
t = _.ax_heatmap.set_xticklabels(_.ax_heatmap.get_xmajorticklabels(), fontsize = 16)
t = _.ax_heatmap.set_yticklabels(_.ax_heatmap.get_ymajorticklabels(), fontsize = 16, rotation=0)
plt.savefig(fig_filename, dpi=300, bbox_inches='tight')
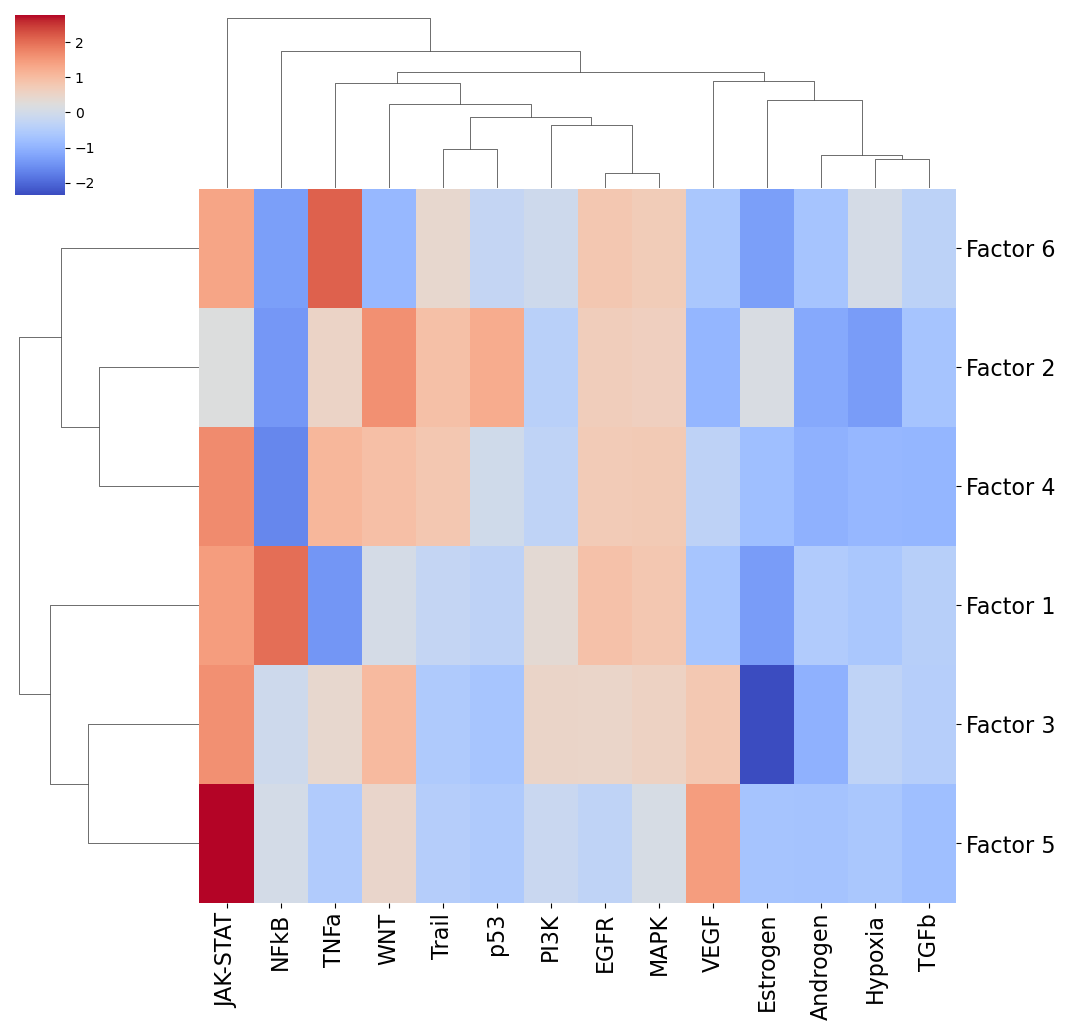
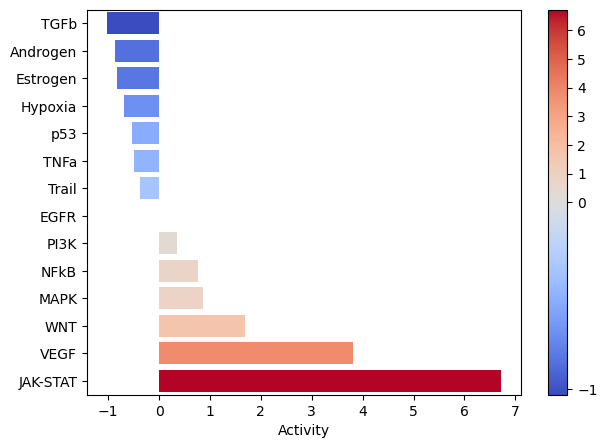
Let’s zoom in on Factor 5, which was most associated with Interferon-beta stimulation.
[48]:
selected_factor = 'Factor 5'
fig_filename = output_folder + '/PROGENy-{}.pdf'.format(selected_factor.replace(' ', '-'))
dc.plot_barplot(estimate,
selected_factor,
vertical=True,
cmap='coolwarm',
save=fig_filename)